Unlocking Sugar: Engineering the Conversion Step in Spirit Production
The previous article discussed processing base materials to expose the inner starches. This article will discuss the next step in the spirits production flow; conversion.
Technical Background
If the sugars are still in a complex form, they must be converted to simple sugars for yeast consumption. Typically, this step involves heat and/or enzymes to convert carbohydrates into simple sugars.
For agave in tequila production, cut agave hearts are slowly steam heated in an autoclave or traditional brick oven. “The low pH (4.5) together with the high temperature hydrolyzes inulin and other components of the plant” (Jacques). Inulin is mainly hydrolyzed into fructose. Softened cooked agave hearts are further processed into a pulp. Syrup run off from the cooking process along with the milled pulp or juice create the sugary wort for fermentation.
The conversion from starch to simple sugars requires more background knowledge. Starch is a carbohydrate, composed of chains of carbon, hydrogen, and oxygen. Carbohydrates are named after their chemical composition: carbo (carbon) and hydrate (water) and follow the formula Cn(H2O)n or (CH2O)n. Starch granules are composed of 2 forms of glucose chains: amylose and amylopectin. Amylose are linear, coiled chains of glucose and amylopectin are branched chains of glucose. Different grains and potatoes have different percentages of amylose and amylopectin. “Natural starches consist of about 10 to 30% amylose and 70 to 90% amylopectin” (Ogori).
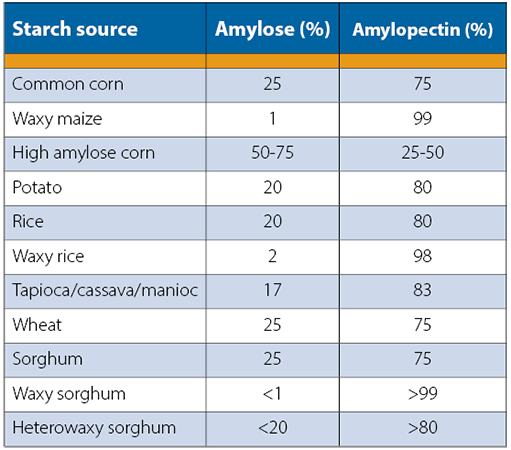
Table 1: Spirit bases composed of starches have different compositions of amylose and amylopectin chains. Table per (Jacques).
The chemical structure of the glucose chains is held together with hydrogen bonds. These hydrogen bonds between the starch molecules and adjacent molecules make the starch resistant to water and enzymes at ambient temperature. Imagine flakes of oatmeal floating in room temperature water, there isn’t much of a reaction happening.
As heat is applied to the starch and water mixture, the starch begins to absorb water and expand as the heat breaks hydrogen bonds of the starch chains. As heating progresses, the enlarged starch granules allow the linear amylose chains to leave the starch granule and mix with the surrounding water, increasing the viscosity of the mash. Eventually, the starch granules expand to the point where they burst like a balloon, exposing all the inner glucose chains.
The beginning of the distillery mashing process is much like making oatmeal at home. Pouring cold water on oats will not provide a thick, delicious breakfast. The water must be hot to allow the starch to absorb the water and gelatinize.
In a distillery, the process of mashing adds hot water to ground starch bases which will result in a thick gelatinous oatmeal-like fluid. This is the desired result of starch hydrolysis in the kitchen when thickening soups or making gum drops. In a distillery, the sugars need to be further cleaved and the liquid needs to be thin enough to pump. The addition of amylase (alpha and beta) enzymes break down the liberated long chains of amylose and amylopectin into smaller glucose chains called dextrins. Alpha amylase tolerates higher temperatures and breaks down starch chains more randomly and from within the chain (as opposed to the end). This process is also called liquification as the viscosity decreases with the addition of α-amylase. Beta amylase tolerates lower temperatures and breaks down chains methodically from the end. In mash bills containing malted grains, the enzymatic activity could come from naturally occurring enzymes in the malt. Commercial amylase is added or supplemented if natural enzymes are not present in the mash.
Neither amylase breaks down the branched connections of the amylopectin. A second enzyme, glucoamylase, is added to break the branched connections of amylopectin as well as break down dextrins into fermentable glucose molecules. The mash is now ready for fermentation.
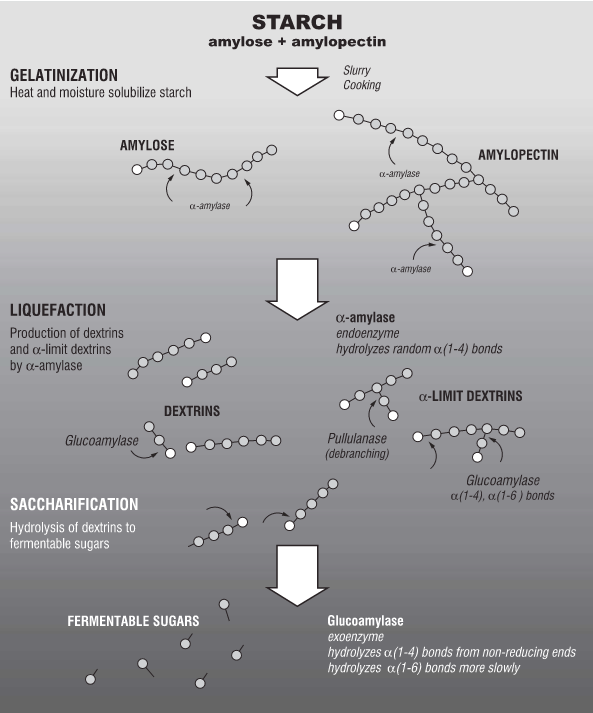
Figure 1: Carbohydrates are composed of a mixture of amylose and amylopectin chains. Amylase and Glucoamylase enzymes break those chains into shorter molecules during the mash process, which will make simple sugars accessible to the yeast during fermentation. Figure per (Jacques).
Heat for conversion can be provided by several methods including: indirect heat using a hot fluid in the vessel jacket, the vessel can be directly heated with a flame or burner, or direct steam injection to the cooking vessel.
The result of this step is a fermentable fluid. The fluid may contain more organic material from the base such as pulp, skins, husks, etc. or the excess organic matter could be removed at this stage of the process leaving a liquid with less particulates to be fermented. Once removed, either after conversion, after fermentation, or after distillation, the excess organic matter can potentially be dried and used as animal feed.
Fermentation happens between 70-90°F but starch conversion takes place at a higher temperature. This means the mash must be heated, and then cooled, before fermentation begins. The conversion step is likely the first of several steps within a distillery where the temperature of the product needs to be increased or decreased. This creates an opportunity for heat integration in a distillery. For example, hot mash can be used to heat water for clean in place (CIP) or to pre-heat the fermented fluid before it is distilled.
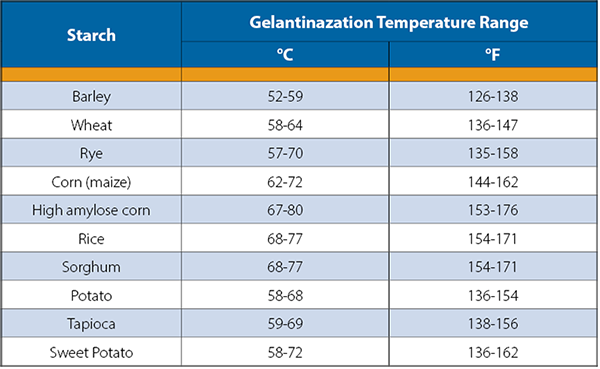
Table 2: Starch bases have different gelatinization temperatures. Table from (Strickland)
How Matrix can help
Matrix can provide deliverables that are traditional to industrial projects: tank sizing, hydraulic models, relief design, equipment specifications, process and instrumentation diagrams (P&IDs), etc., as well as deliverables with extra benefit to the distilling industry.
For example, Matrix has developed simple distillery throughput models to identify capacity limiting steps within production (mashing, fermentation, distillation). The quantitative model is a valuable tool to determine where future expansion and investments will be most beneficial.
Sustainability goals are also becoming more significant in the distillery industry. Matrix has experience in utility design including heat integration. Matrix can support a distillery’s environmental goals to minimize freshwater use, clean and reuse water on site, and design heat transfer systems to maximize heat generation or dissipation throughout the process and facility.
The next article in the series will discuss digesting the sugary liquid into alcohol.
References:
Ogori, A. Taofeek. "Starch Chemistry and Application." Journal of Food Technology and Preservation 2022.
Jacques, Lyons, Kelsall. The Alcohol Textbook 4th Edition. Nottingham: Nottingham University Press, 2003.
Strickland, Matt. https://distilling.com/distillermagazine/mash-chemistry-101/. 27 December 2019. 17 January 2025.
© Matrix Technologies, Inc.